Abstract
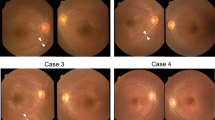
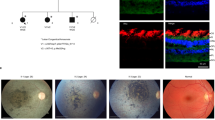
In addition to its activity in nicotinamide adenine dinucleotide (NAD+) synthesis, the nuclear nicotinamide mononucleotide adenyltransferase NMNAT1 acts as a chaperone that protects against neuronal activity–induced degeneration. Here we report that compound heterozygous and homozygous NMNAT1 mutations cause severe neonatal neurodegeneration of the central retina and early-onset optic atrophy in 22 unrelated individuals. Their clinical presentation is consistent with Leber congenital amaurosis and suggests that the mutations affect neuroprotection of photoreceptor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Accession codes
References
den Hollander, A.I. et al. Prog. Retin. Eye Res. 27, 391–419 (2008).
Sergouniotis, P.I. et al. Am. J. Hum. Genet. 89, 183–190 (2011).
Estrada-Cuzcano, A. et al. Invest. Ophthalmol. Vis. Sci. 52, 834–839 (2011).
Zhou, T. et al. J. Biol. Chem. 277, 13148–13154 (2002).
Perrault, I. et al. Hum. Mutat. 28, 416 (2007).
Raffaelli, N. et al. Biochem. Biophys. Res. Commun. 297, 835–840 (2002).
Berger, F. et al. J. Biol. Chem. 280, 36334–36341 (2005).
Conforti, L. et al. FEBS J. 278, 2666–2679 (2011).
Zhai, R.G. et al. PLoS Biol. 4, e416 (2006).
Mack, T.G. et al. Nat. Neurosci. 4, 1199–1206 (2001).
Coleman, M.P. et al. Annu. Rev. Neurosci. 33, 245–267 (2010).
Avery, M.A. et al. J. Cell Biol. 184, 501–513 (2009).
Feng, Y. et al. Protein Cell 1, 237–245 (2010).
Sasaki, Y. et al. J. Neurosci. 29, 5525–5535 (2009).
Lau, C. et al. J. Biol. Chem. 285, 18868–18876 (2010).
Acknowledgements
We are grateful to the individuals with Leber congenital amaurosis and their families for their participation in this study. We thank P. Debruyne for making available the fundus pictures of subject P4. This research was supported by the Association Retina France (grant to J.-M.R.) and the Assistance Publique–Hôpitaux de Paris (AP-HP; grant PHRC National-2009 AOM 09058/P081257 to J.K.). We also thank the Association Retina France for supporting the 100 Exomes project of the French Research Network aiming to speed the genetic analysis of inherited retinal diseases through whole-exome resequencing. Written consent was obtained from participants or legally authorized representatives. The study was conducted in strict adherence to the tenets of the Declaration of Helsinki and was approved by the Comité de Protection des Personnes Ile-de-France II.
Author information
Authors and Affiliations
Contributions
J.-M.R., J.K., H.D. and A.M. designed the study. I.P., S.H. and P.N. analyzed data from exome sequencing. I.P. and S.H. performed and analyzed data from Sanger sequencing of affected individuals and controls. M.N., N.D., L.F.-T. and S.G. performed linkage analyses at the LCA loci and Sanger sequencing of candidates to select the subjects to be screened for NMNAT1 mutations. O.X. performed and analyzed the LD analyses at the NMNAT1 locus. V.S. performed the three-dimensional structural analysis of NMNAT1 missense variants. J.K., H.D., X.Z., S.D.-D., C.E., A.G., A.D., G.L.M., C.H., E.S., P.C. and O.R. provided and analyzed clinical material from subjects. All authors contributed to the manuscript written by J.-M.R. and J.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Perrault, I., Hanein, S., Zanlonghi, X. et al. Mutations in NMNAT1 cause Leber congenital amaurosis with early-onset severe macular and optic atrophy. Nat Genet 44, 975–977 (2012). https://doi.org/10.1038/ng.2357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2357
This article is cited by
-
Spectrum of variants associated with inherited retinal dystrophies in Northeast Mexico
BMC Ophthalmology (2024)
-
Molecular background of Leber congenital amaurosis in a Polish cohort of patients—novel variants discovered by NGS
Journal of Applied Genetics (2023)
-
Clinical features and genetic spectrum of NMNAT1-associated retinal degeneration
Eye (2022)
-
Novel gene variants in Polish patients with Leber congenital amaurosis (LCA)
Orphanet Journal of Rare Diseases (2020)
-
Genetic spectrum of retinal dystrophies in Tunisia
Scientific Reports (2020)