Abstract
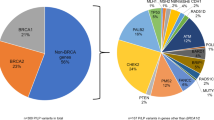
An increasing number of mismatch-repair (MMR) gene mutations have been identified in hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome. This study presents the population-based Danish MMR gene mutation profile, which contains 138 different MMR gene alterations. Among these, 88 mutations in 164 families are considered pathogenic and an additional 50 variants from 76 families are considered to represent variants of unknown pathogenicity. The different MMR genes contribute to 40% (MSH2), 29% (MLH1), and 22% (MSH6) of the mutations and the Danish population thus shows a considerably higher frequency of MSH6 mutations than previously described. Although 69/88 (78%) pathogenic mutations were present in a single family, previously recognized recurrent/founder mutations were causative in 75/137 (55%) MLH1/MSH2 mutant families. In addition, the Danish MLH1 founder mutation c.1667+2_1667_+8TAAATCAdelinsATTT was identified in 14/58 (24%) MLH1 mutant families. The Danish Lynch syndrome population thus demonstrates that MSH6 mutations and recurrent/founder mutations have a larger contribution than previously recognized, which implies that the MSH6 gene should be included in routine diagnostics and suggests that directed analysis of recurrent/founder mutations may be feasible e.g. in families were diagnostic material is restricted to archival tissue.


Similar content being viewed by others
References
Bronner CF, Baker SM, Morrison PT et al (1994) Mutations in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colorectal cancer. Nature 368:258–261. doi:10.1038/368258a0
Fishel R, Lescoe MK, Rao MR et al (1993) The human mutator gene homologue MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75:1027–1038. doi:10.1016/0092-8674(93)90546-3
Leach FS, Nicolaides NC, Papadopoulos N et al (1993) Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75:1215–1225. doi:10.1016/0092-8674(93)90330-S
Papadopoulos N, Nicolaides NC, Wei YF et al (1994) Mutation of a mutL homolog in hereditary colon cancer. Science 263:1625–1629. doi:10.1126/science.8128251
Woods MO, Williams P, Careen A et al (2007) A new variant database for mismatch repair genes associated with Lynch syndrome. Hum Mutat 28:669–673. doi:10.1002/humu.20502
Peltomaki P, Vasen H (2004) Mutations associated with HNPCC predisposition – update of ICG-HNPCC/INSiGHT mutation database. Dis Markers 20:269–276
Vasen HF, Watson P, Mecklin J-P, Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116:1453–1456. doi:10.1016/S0016-5085(99)70510-X
Bisgaard ML, Jäger AC, Myrhoj T, Bernstein I, Nielsen FC (2002) Hereditary non-polyposis colorectal cancer (HNPCC): phenotype–genotype correlation between patients with and without identified mutation. Hum Mutat 20:20–27. doi:10.1002/humu.10083
Katballe N, Christensen M, Wikman FP, Orntoft TF, Laurberg S (2002) Frequency of hereditary non-polyposis colorectal cancer in Danish colorectal cancer patients. Gut 50:43–51. doi:10.1136/gut.50.1.43
Roncari B, Pedroni M, Maffel S et al (2007) Frequency of constitutional MSH6 mutations in a consecutive series of families with clinical suspicion of HNPCC. Clin Genet 72:230–237. doi:10.1111/j.1399-0004.2007.00856.x
Umar A, Boland CR, Terdiman JP et al (2004) Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96:261–268
Mitchell RJ, Farrington SM, Dunlop MG, Campbell H (2002) Mismatch repair genes hMLH1 and hMSH2 in colorectal cancer: a HuGE review. Am J Epidemiol 15:865–902
Wijnen J, Meera Khan P, Vasen H et al (1996) Majority of hMLH1 mutations responsible for hereditary nonpolyposis colorectal cancer cluster at the exonic region 15–16. Am J Hum Genet 58:3000–3007
Baudhuin LM, Ferber MJ, Winters JL et al (2005) Characterization of hMLH1 and hMSH2 gene dosage alterations in Lynch syndrome patients. Gastroenterology 129:846–854. doi:10.1053/j.gastro.2005.06.026
Grabrovski M, Mueller-Koch Y, Grasbon-Frodl E et al (2005) Deletions account for 17% of the pathogenetic germline alterations in MLH1 and MSH2 in hereditary nonpolyposis colorectal cancer (HNPCC) families. Genet Test 9:138–146. doi:10.1089/gte.2005.9.138
Taylor CF, Charlton RS, Burn J, Sheridan E, Taylor GR (2003) Genomic deletions in MSH2 or MLH1 are frequent cause of hereditary non-polyposis colorectal cancer: identification of novel and recurrent deletions by MLPA. Hum Mutat 22:428–433. doi:10.1002/humu.10291
Foulkes WD, Thiffault I, Gruber SB et al (2002) The founder mutation MSH2*1906G>C is an important cause of hereditary nonpolyposis colorectal cancer in Askenazi jewish population. Am J Hum Genet 71:1395–1412. doi:10.1086/345075
Jäger AC, Rasmussen M, Bisgaard HC et al (2001) HNPCC mutations in the human DNA mismatch repair gene hMLH1 influence assembly of hMutLa and hMLH1-hEXO1 complexes. Oncogene 20:3590–3595. doi:10.1038/sj.onc.1204467
Kondo E, Suzuki H, Horii A, Fukushige S (2003) A yeast two-hybrid assay provides a simple way to evaluate the vast majority of hMLH1 germ-line mutations. Cancer Res 63:3302–3308
Ollila S, Sarantaus L, Kariola R et al (2006) Pathogenicity of MSH2 missense mutations is typically associated with impaired repair capability of the mutated protein. Gastroenterology 131:1408–1417. doi:10.1053/j.gastro.2006.08.044
Takahashi M, Shimodaira H, Andreutti-Zaugg C et al (2007) Functional analysis of human MLH1 variants using yeast and in vitro mismatch repair assays. Cancer Res 67:4595–4604. doi:10.1158/0008-5472.CAN-06-3509
Raevaara TE, Gerdes A-M, Lönnqvist KE et al (2004) HNPCC mutation MLH1 P648S makes the functional protein unstable, and homozygosity predisposes to mild neurofibromatosis. Genes Chromosomes Cancer 40:261–265. doi:10.1002/gcc.20040
Desai DC, Lockman JC, Chadwick RB et al (2000) Recurrent germline mutation in MSH2 arises frequently de novo. J Med Genet 37:646–652. doi:10.1136/jmg.37.9.646
Froggatt NJ, Geen J, Bassett C et al (1999) A common MSH2 mutation in English and North American HNPCC families: origin, phenotypic expression, and sex specific differences in colorectal cancer. J Med Genet 36:97–102
Mangold E, Pagenstecher C, Friedl W et al (2005) Spectrum and frequencies of mutations in MSH2 and MLH1 identified in 1,721 German families suspected of hereditary nonpolyposis colorectal cancer. Int J Cancer 116:692–702. doi:10.1002/ijc.20863
Miyaki M, Konishi M, Muraoka M et al (1995) Germ line mutations of hMSH2 and hMLH1 genes in Japanese families with hereditary nonpolyposis colorectal cancer (HNPCC): usefulness of DNA analysis for screening and diagnosis of HNPCC patients. J Mol Med 73:515–520. doi:10.1007/BF00198903
Wagner A, Barrows A, Wijnen JT et al (2003) Molecular analysis of hereditary non-polyposis colorectal cancer in the United States: High mutation detection rates among clinically selected families and characterization of an American founder genomic deletion of the MSH2 gene. Am J Hum Genet 72:1088–1100. doi:10.1086/373963
Nystrom-Lathi M, Kristo P, Nicolaides NC et al (1995) Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nat Med 1:1203–1206. doi:10.1038/nm1195-1203
Sun S, Greenwood CM, Thuffault I et al (2005) The HNPCC associated MSH2*1906G-C founder mutation probably originated between 1440 and 1715 in the Ashkenazi Jewish population. J Med Genet 42:766–768. doi:10.1136/jmg.2005.030999
Jäger AC, Bisgaard ML, Myrhöy T et al (1997) Reduced frequency of extracolonic cancers in hereditary nonpolyposis colorectal cancer families with monoallelic hMLH1 expression. Am J Hum Genet 61:129–138. doi:10.1086/513896
Malander S, Rambech E, Kristoffersson U et al (2006) The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol 101:238–243. doi:10.1016/j.ygyno.2005.10.029
Lagerstedt RK, Liu T, Vandrovcova J et al (2007) Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst 99:291–299. doi:10.1093/jnci/djk051
Hendriks YM, Wagner A, Morreau H et al (2004) Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology 127:17–25. doi:10.1053/j.gastro.2004.03.068
Acknowledgements
Financial support was granted from the Danish Cancer Society and from the Hvidovre University Hospital. We would also like to acknowledge surgeons, pathologists, and geneticists for identifying these families and reporting data to the national HNPCC-register.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nilbert, M., Wikman, F.P., Hansen, T.V.O. et al. Major contribution from recurrent alterations and MSH6 mutations in the Danish Lynch syndrome population. Familial Cancer 8, 75–83 (2009). https://doi.org/10.1007/s10689-008-9199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-008-9199-3