Abstract.
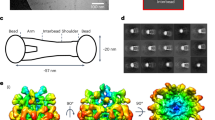
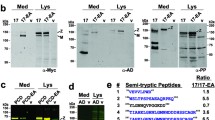
Mutations in the fibrillin-1 gene (FBN1) cause Marfan syndrome (MFS), an autosomal dominant disorder of connective tissue with highly variable clinical manifestations. FBN1 contains 47 epidermal growth factor (EGF)-like modules, 43 of which display a consensus sequence for calcium binding (cbEGF). Calcium binding by cbEGF modules is thought to be essential for the conformation and stability of fibrillin-1. Missense mutations in cbEGF modules are the most common mutations found in MFS and generally affect one of the six highly conserved cysteines or residues of the calcium-binding consensus sequence. We have generated a series of recombinant fibrillin-1 fragments containing six cbEGF modules (cbEGF nos. 15–20) with various mutations at different positions of cbEGF module no. 17, which is known to contain a cryptic cleavage site for trypsin. A mutation affecting a residue of the calcium-binding consensus sequence (K1300E) found in a patient with relatively mild clinical manifestations of classic MFS caused a modest increase in susceptibility to in vitro proteolysis by trypsin, whereas a mutation affecting the sixth cysteine residue of the same cbEGF module (C1320S) reported in a severely affected patient caused a dramatic increase in susceptibility to in vitro proteolysis by trypsin. A mutation at the cryptic cleavage site for trypsin abolished sensitivity of wild-type fragments and fragments containing K1300E to trypsin proteolysis. Whereas the relevance of in vitro proteolysis to the in vivo pathogenesis of MFS remains unclear, our findings demonstrate that individual mutations in cbEGF modules can affect these modules differentially and may suggest an explanation for some genotype-phenotype relationships in MFS.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Booms, P., Tiecke, F., Rosenberg, T. et al. Differential effect of FBN1 mutations on in vitro proteolysis of recombinant fibrillin-1 fragments. Hum Genet 107, 216–224 (2000). https://doi.org/10.1007/s004390000368
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004390000368